Stop suicide, save a life
New data from the Centers for Disease Control and Prevention shows that suicide rates have risen to over 30% in the US since 1999.[1] Since COVID-19 began, suicidal ideation in the US has more than doubled, with younger adults, racial/ethnic minorities, essential workers and unpaid adult caregivers experiencing disproportionately worse effects.[2] As suicide has reached crisis-level proportions in our nation, it’s time to recognize suicide as a public health crisis and learn about the warning signs and the skills needed to save a life.
Know the warning signs of suicide
It is hard to tell whether a person is thinking of suicide. Most people who take their own life show one or more warning signs, either through what they say or do.
- Feelings: Expressing hopelessness, talking about suicide or having no reason to live, showing moods such as depression, anxiety, irritability
- Actions: Showing severe/overwhelming pain or distress, using drugs or alcohol, searching for ways to end their life
- Changes: Withdrawing from activities, isolating from friends and family, sleeping more or less
- Threats: Talking about, writing about or making plans to kill themselves
- Situations: Going through stressful situations including loss, change, personal humiliation or difficulties at home, school or with the law
Take action to prevent suicide
Suicide remains the second leading cause of death among Americans between the ages 10 and 34, according to the CDC.[3] It is a major health crisis—and preventable. When someone says they are thinking about suicide or says things that sound as if they are considering suicide, it is important to pay attention and take action. Suicide is often preventable.
- Ask and listen: “Are you thinking about killing yourself?” is not an easy question, however, a study by the National Institute of Mental Health shows considering suicide may reduce rather than increase suicidal thoughts. Be willing to listen and discuss their feelings.
- Keep them safe: Reducing a person’s access to highly lethal objects or places is an important part of suicide prevention. Asking if the at-risk person has a plan and removing access to lethal means can make a difference.
- Get them help: Connect with a trusted family member, friend or mental health professional. Call the National Suicide Prevention Lifeline’s (1-800- 273-TALK (8255)) and the Crisis Text Line’s number (741741). Save these numbers in your phone so they’re there when you need them.
- Stay connected: Staying in touch after a crisis or discharge from care can make a difference. Let them know they matter and you care. Leave a message, send a text or call them.
For more information and helpful resources, visit MagellanHealthcare.com/Prevent-Suicide.
If you are in crisis or considering suicide, or if someone you know is currently in danger, please dial 911 immediately.
[1] https://www.nimh.nih.gov/health/statistics/suicide



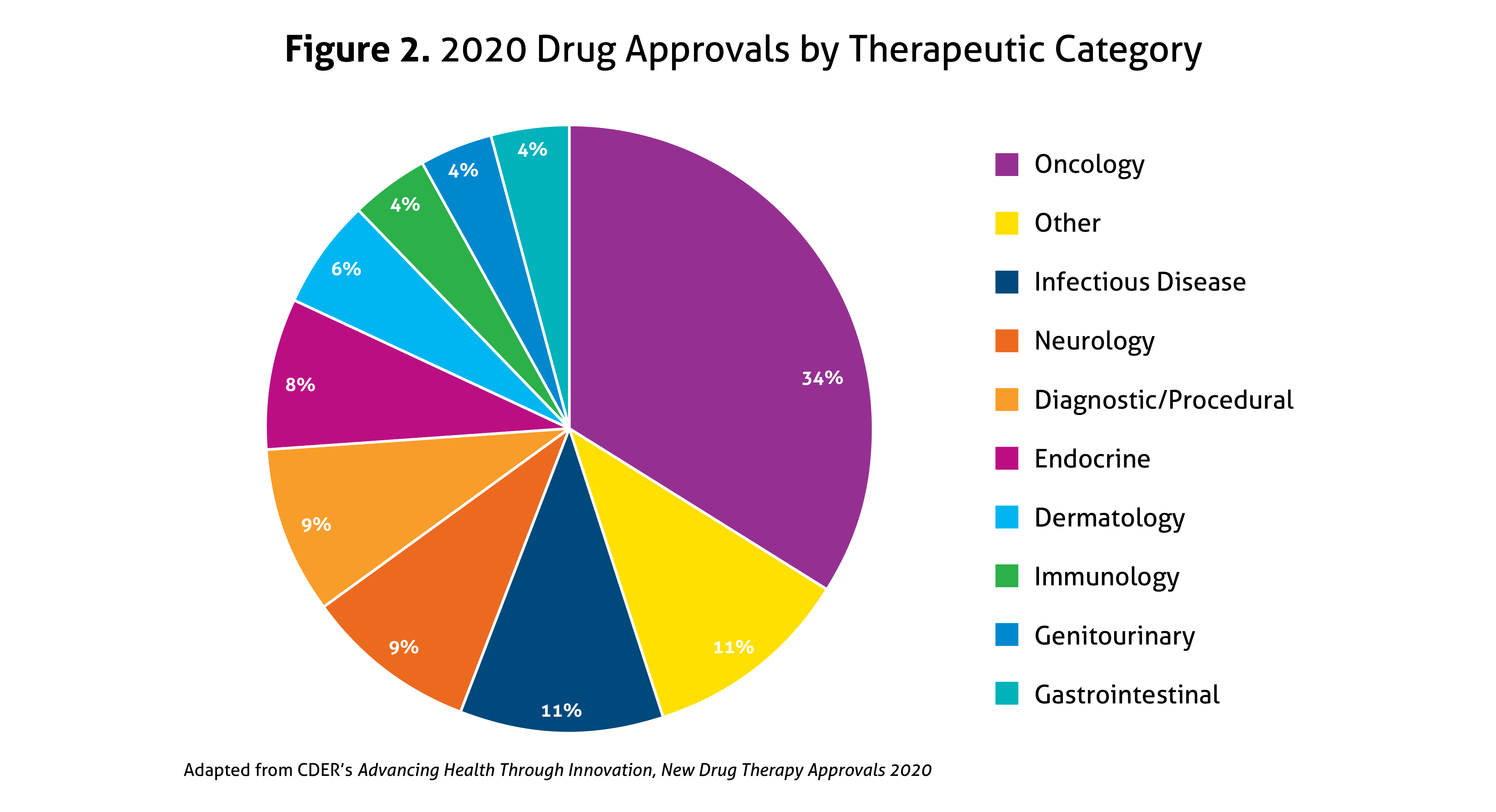
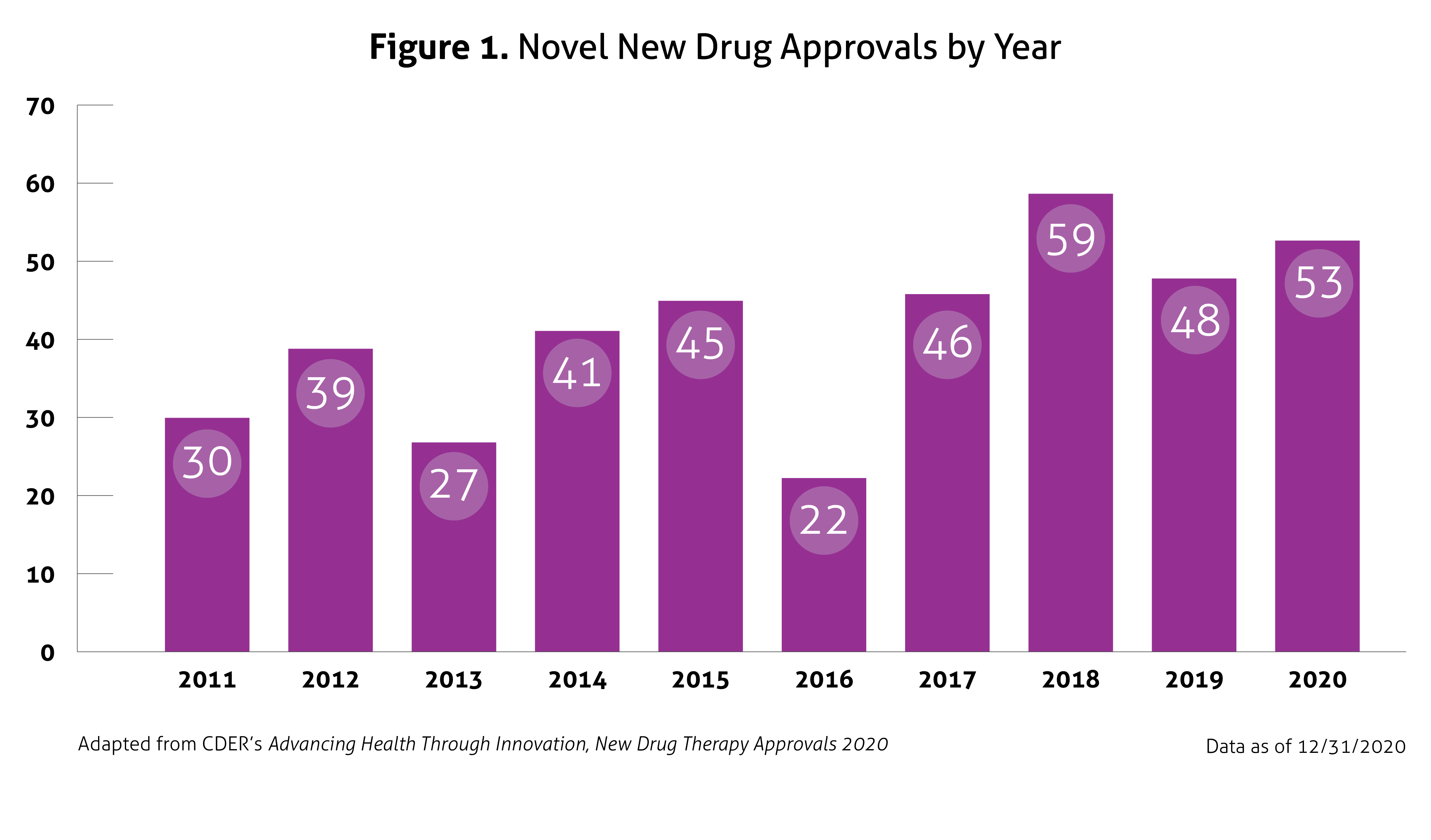 Despite the ongoing pandemic the FDA continued their strategic initiatives to expedite the safe review of treatments in 2020. With the unprecedented challenges incurred in 2020, the FDA acknowledged that maintaining their commitment to bringing forth innovative therapies was difficult. Remarkably, the numbers reported by the FDA do not include the several emergency use authorizations (EUAs) issued by the FDA for COVID-19.
Despite the ongoing pandemic the FDA continued their strategic initiatives to expedite the safe review of treatments in 2020. With the unprecedented challenges incurred in 2020, the FDA acknowledged that maintaining their commitment to bringing forth innovative therapies was difficult. Remarkably, the numbers reported by the FDA do not include the several emergency use authorizations (EUAs) issued by the FDA for COVID-19.